Abstract
Introduction: Durability of minimal residual disease (MRD) based on bone marrow examination is associated with a progression-free survival (PFS) benefit when it is undetected for >6-12 months. However, MRD assessment is limited by the heterogeneous involvement of bone marrow and invasive nature of the procedure. In contrast, Mass-Fix is a mass spectrometry based blood test that can detect small amounts of monoclonal protein. Herein, we hypothesize that achieving sustained Mass-Fix negativity for at least 6 or 12 months, improves outcomes compared to patients with nonsustained Mass-Fix negativity. In addition, we assess the clinical outcome of patients with loss of Mass-Fix negativity.
Methods: This retrospective cohort study included consecutive patients seen at Mayo Clinic Rochester with newly diagnosed multiple myeloma (MM). Patients achieving at least very good partial response, and who had serial Mass-Fix testing every 6-12 months were included in this study. MRD in bone marrow aspirate was measured by flow cytometry (threshold 1 tumor cell/105 white blood cells). The Kaplan Meier or Simon-Makuch methods were used to graph the survival function. Progression-free survival was defined from diagnosis or the landmark point (2 years after diagnosis) to progression or death from any cause.
Results: We identified 443 patients with NDMM, between May 1, 2017 and May 31, 2020. Among these, 274 patients had marrow MRD examination during their follow-up. With a median follow-up of 41 months from diagnosis, 56% of patients achieved Mass-Fix negativity, 43% marrow MRD negativity, and 23% achieved both Mass-Fix and MRD negativity. Most patients received a bortezomib-lenalidomide-dexamethasone induction followed by autologous transplantation (73%).
In a 2 year landmark analysis, patients achieving Mass-Fix negativity had a better PFS at 2-years from landmark (4 years from diagnosis) than patients who did not (86.7% vs. 70.0% , p<0.001). Similarly, in a landmark analysis, patients with negative marrow MRD had a better PFS than those with positive MRD (88.1% vs. 62.5%, p=0.027). However, overall survival was similar between patients achieving Mass-Fix or MRD negativity and those who did not.
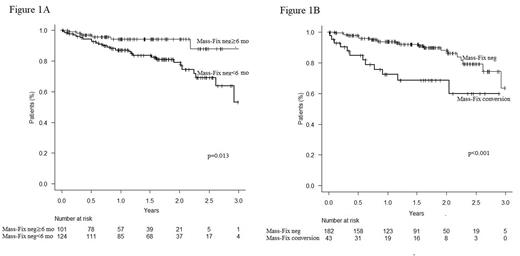
In patients with Mass-Fix negativity, 76% and 71% achieved sustained Mass-Fix negativity for ≥6 and ≥12 months, respectively. In a landmark analysis, a significant difference in 2-year PFS was observed between patients with sustained Mass-Fix negativity ≥6 months and < 6 months (94.1% vs. 78.9%, p=0.013, Figure 1A). Patients with sustained Mass-Fix negativity≥12 months and < 12 months had a better 2-year PFS in a landmark analysis (94.4 vs. 80.2, p=0.019). Similarly, in a landmark analysis, patients with sustained marrow MRD negativity for ≥12 months had a better 2-year PFS from the landmark (4 years from diagnosis) than patients with MRD negativity for <12 months (91.8% vs. 72.8%, p<0.001). Patients achieving both sustained Mass-Fix and marrow negativity for ≥6 months had a better 2-year PFS from the landmark than patients who did not in a landmark analysis (91.5% vs. 73.1%, p=0.001).
In our cohort, 33% of patients achieving Mass-Fix negativity had a subsequent loss of Mass-Fix negativity by 4 years; median duration of Mass-Fix negativity was 27 months (95% CI 25-30 months). Nearly half of patients with loss of Mass-Fix negativity (39/81) had eventually progressive disease (PD) according to IMWG criteria. In a 2-year landmark analysis, 2-year PFS (4 years from diagnosis) was better in patients with ≥2 consecutive negative Mass-Fix than in patients with loss of Mass-Fix negativity (88.1% vs. 68.6%, p<0.001, Figure 1B). Patients with loss of Mass-Fix negativity after the first year following diagnosis had a better 2-year overall survival than patients with conversion before 1 year in a landmark analysis (100.0% vs. 90.6%, p=0.045).
Finally, patients who achieved sustained Mass-Fix negativity for ≥6 months had a better 4-year PFS than patients with loss of sustained Mass-Fix negativity with Simon-Makuch method (94.0% vs. 28%, p<0.001).
Conclusions: In summary, this study demonstrates that sustained Mass-Fix negativity may represent a robust evaluation of prognosis and disease control. Moreover, loss of Mass-Fix negativity is associated with a worse outcome and could be used as a marker for earlier treatment interventions in MM.
Disclosures
Dispenzieri:Alynlam, Pfizer, Takeda, and BMS: Research Funding; Oncopeptides, and Sorrento: Other: Data monitoring safety committee; Janssen: Membership on an entity's Board of Directors or advisory committees. Kapoor:Sanofi: Honoraria, Research Funding; X4 Pharmaceuticals: Honoraria; Regeneron: Research Funding; Amgen: Research Funding; Ichnos: Research Funding; Loxo: Research Funding; Karyopharma: Research Funding; BMS: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Research Funding; Casma: Honoraria; Pharmacyclics: Honoraria; Imedex: Honoraria; GSK: Honoraria; Cellectar: Honoraria; Oncopeptides: Honoraria. Dingli:Takeda Pharmaceuticals: Consultancy; Sanofi S.A.: Consultancy; Novartis: Consultancy; Janssen Pharmaceuticals: Consultancy; GlaxoSmithKline: Consultancy; Bristol Myers Squibb: Consultancy; Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals: Consultancy. Gertz:Ionis/Akcea: Other: personal fees; Prothena: Other: personal fees; Sanofi: Other: personal fees; Janssen: Other: personal fees; Aptitude Healthgrants: Other: personal fees; Ashfield: Other: personal fees; Juno: Other: personal fees; Physicians Education Resource: Other: personal fees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: personal fees from Data Safety Monitoring Board; Johnson & Johnson: Other: personal fees; Celgene: Other: personal fees; Research to Practice: Other: personal fees; Sorrento: Other: personal fees; i3Health: Other: Development of educational materials for i3Health. Kourelis:Novartis: Research Funding. Lacy:Celgene: Research Funding. Leung:Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Lin:Vineti: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Sorrento: Consultancy; Juno: Consultancy; Janssen: Consultancy, Research Funding; Novartis: Consultancy; Merck: Research Funding; Takeda: Research Funding; Legend: Consultancy; Celgene: Consultancy, Research Funding; Gamida Cell: Consultancy; Bluebird Bio: Consultancy, Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal